Tiny machines in bacteria could help make new medicines
Scientists work to unlock the full potential of biological machines that can have a huge impact on human health.
By Erin MatthewsProtein crystals of the NRPS oxidase complex.
With the help of the Canadian Light Source (CLS) at the University of Saskatchewan, researchers from McGill University are trying to unlock the full potential of tiny biological machines that can have a huge impact on human health.
These machines — known as nonribosomal peptide synthetases (NRPSs) — are critical for creating therapeutic molecules found in a variety of medicines, from antibiotics to immunosuppressants.
“Microbes like bacteria and fungi have these NRPS machines that are responsible for making molecules that act as important drugs and therapeutics,” said Camille Fortinez, a recent PhD graduate of McGill’s Department of Biochemistry and lead author on a paper published in Nature Communications.
Understanding how NRPSs create therapeutics will help researchers to design new drugs and to combat global health issues like antibiotic resistance.
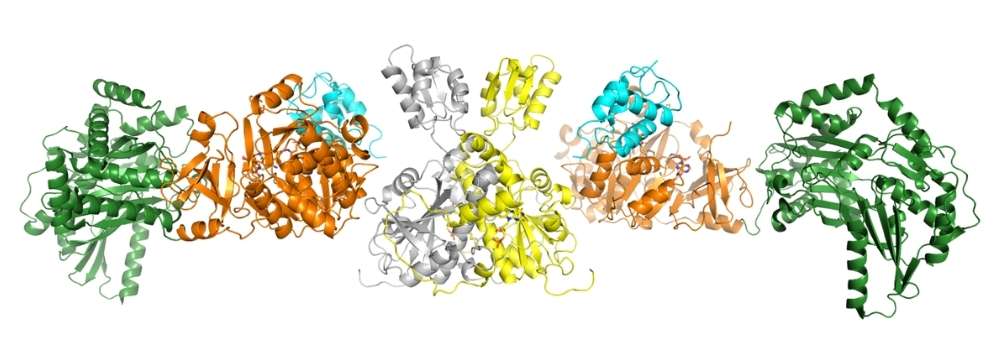
With the help of the CMCF beamline at the CLS, Martin Schmeing, Professor of Biochemistry at McGill, and Fortinez were able to look at one common NRPS machine in great detail.
“CLS has allowed us to get a really high-resolution diffraction of our NRPS crystals,” Fortinez said. “This high resolution is really integral for allowing us to answer questions and better understand the NRPS.”
The team analyzed an NRPS found in many bacteria that helps generate a chemical that kills algae. In the process, Fortinez and Schmeing discovered that a separate enzyme is responsible for a crucial stage in the production of this algae-killing compound.
The NRPS machines that the team studied could help to develop new therapeutics and the algae-killing compound might be modified to kill bacteria that threaten our health. The researchers are hopeful that the detailed data they collected will help to lead the way.
“The CLS is a wonderful national resource that we are deeply indebted to and it's a really important resource to keep Canadian science doing as well as we are doing,” Schmeing said.
Fortinez, Camille Marie, Kristjan Bloudoff, Connor Harrigan, Itai Sharon, Mike Strauss, and T. Martin Schmeing. "Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module." Nature Communications 13, no. 1 (2022): 1-13. DOI: 10.1038/s41467-022-28221-y.
Photos: Synchrotron | CMCF | Schmeing, Fortinez, samples
Media Relations:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
