A new generation of anti-malarial drugs
An international research team used the CLS to help determine the atomic structure of a protein kinase in parasites that cause malaria, which could help create a new generation of anti-malarial drugs.
By Sarath PeirisA mosquito on a person's hand.
Malaria is endemic to large areas of Africa, Asia and South America and annually kills more than 400,000 people, a majority of whom are children under age 5, with hundreds of millions of new infections every year.
Although artemisinin-based drug combinations are available to treat malaria, reports from Southeast Asia of treatment failures are raising concerns about drug resistance spreading to Africa. Fortunately, there is hope on the horizon because there are several new antimalarial drug candidates undergoing clinical testing as well as other promising drug targets that are under investigation.
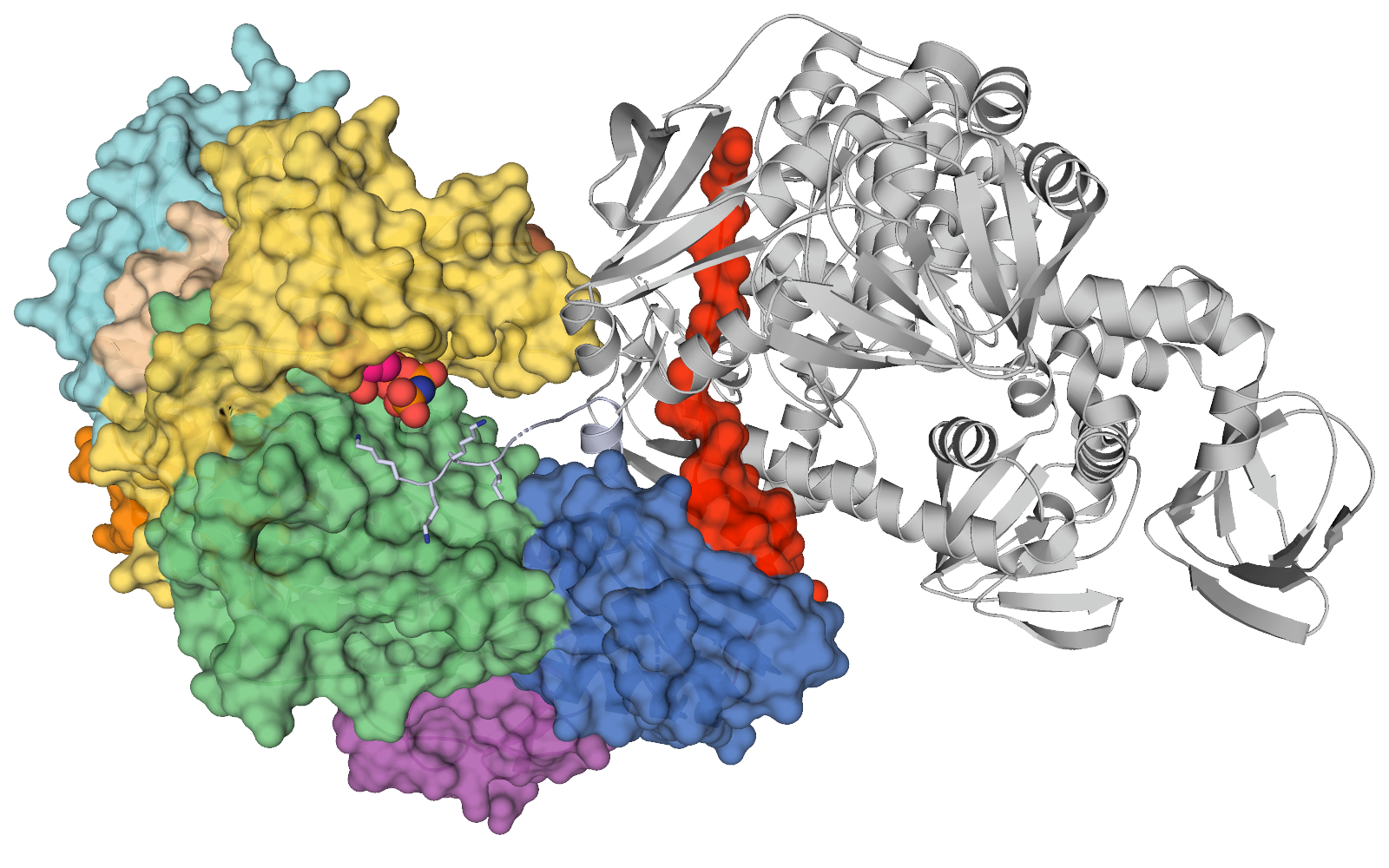
An international research team has for the first time determined the atomic structure of a protein kinase called PKG in Plasmodium parasites that cause malaria—a finding that potentially will help create a new generation of anti-malarial drugs and advance fundamental research.
PKG[i] plays essential roles in the developmental stages of the parasite’s complex life cycle, so understanding its structure is key to developing malaria-fighting therapies that specifically target PKG and not other human enzymes, according to researcher Dr. Charles Calmettes.
“Until now, we didn’t have the structure for PKG from any organism. There are differences between human PKG and malaria PKG, but so far this is the best model we have to understand the mammal version of the protein,” said Calmettes, an assistant professor at INRS-Institut Armand-Frappier in Quebec.
Potent first generation PKG inhibitors have been made that selectively target the parasite PKG, but not the human PKG due to a significant structural difference between the two enzymes in the inhibitor-binding pocket revealed by crystallography studies.[ii]
The Canadian Light Source’s CMCF[iii] beamline at the University of Saskatchewan enabled the researchers to optimize PKG crystals and collect initial datasets for the project.
“Having access to the strong X-ray beam to collect diffraction data and screen protein crystals at the CLS was very important to completing our project,” he said, adding much of the protein structure elucidation was done by researchers at the Structural Genomics Consortium at the University of Toronto.
When an infected mosquito bites a human, malaria parasites are quickly carried to the liver, where they invade liver cells (hepatocytes) and multiply. The hepatocytes burst, releasing tens of thousands of transformed parasites (merozoites) that invade red blood cells. There the merozoites further multiply and rupture the blood cells—a process that produces fever, chills, nausea and other nasty effects of malaria.
A mosquito that feeds on the blood of an infected human will pick up the parasite and transmit the disease to other humans, which continues the deadly cycle.
Previous experiments have shown that several parasite developmental stages are dependent on PKG, which controls the calcium signalling within cells. Calcium signals regulate cell activities, including the precise timing of the release of the merozoites from blood cells, and the replication processes of parasites within the mosquito.
“PKG is a nice drug target because you can target the parasite life cycle stages that cause disease symptoms but also those that transmit disease through mosquitoes thereby preventing the parasite from causing disease in the human host. Experiments have shown that when you add a PKG inhibitor to the transmissible stages (gametocytes), the parasite isn’t able to grow in the mosquito and infect a new host,” said Calmettes.
Other scientists on the research team were from Institut National de la Recherche Scientifique (INRS) in Quebec, Baylor College of Medicine in Houston, London School of Hygiene & Tropical Medicine, University of Toronto Department of Biochemistry, Francis Crick Institute in London, and the Toronto General Hospital Research Institute.
For more information, contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
[i] PKG is short for “cyclic guanosine-3’,5’-monophosphate-dependent protein kinase.”
[ii] Baker et. al. Nat Commun. 2017; PMID: 28874661.
[iii] Canadian Macromolecular Crystallography Facility (CMCF).
