Converting emissions into valuable fuel
Researchers worked improve their technique for converting CO2 into ethanol, a valuable chemical for industrial applications that also reduces emissions.
By Erin MatthewsConfined copper by nitrogen-doped carbon for ethanol production from CO2.
Researchers used the Canadian Light Source (CLS) at the University of Saskatchewan to improve their technique to convert CO2 into ethanol, a valuable chemical that can be used in a variety of industrial applications. Ethanol is also an attractive alternative fuel.
Ethanol has been proven to reduce emissions when compared to gasoline, but the renewable fuel is most often made of corn and wheat so there is a strong interest in non-food production methods. By capturing and converting carbon emissions to ethanol, the fuel’s environmental benefits could be multiplied.
The research team led by Prof. Ted Sargent at the University of Toronto focused on producing chemicals through CO2 conversion—such as ethanol, ethylene and methane—helping to transform harmful greenhouse gases into useful products. The group aims to produce the target chemicals, in this case ethanol, with high outputs and minimal energy inputs.
This ethanol project is sponsored by the Canadian energy company Suncor, which invests in cleaner, renewable fuel sources, the Natural Sciences and Engineering Research Council (NSERC) of Canada, and the CIFAR Bio-Inspired Solar Energy program. If successful, more Canadian renewable fuel like ethanol could be created from greenhouse gases and less from farmed food.
The Science
In a recent paper published in Nature Energy, the team studied ethanol production by developing catalysts through overcoating copper with nitrogen-doped carbon (N-C/Cu). To understand how the catalysts work and provide valuable information for fine-tuning its efficiency, they studied the structure and chemistry of catalysts using the SXRMB beamline at the CLS.
They discovered that gaps below 1 nm between the Cu and N-C layers may act as a reactor during CO2 to ethanol conversion, a property that could be harnessed to maximize output.
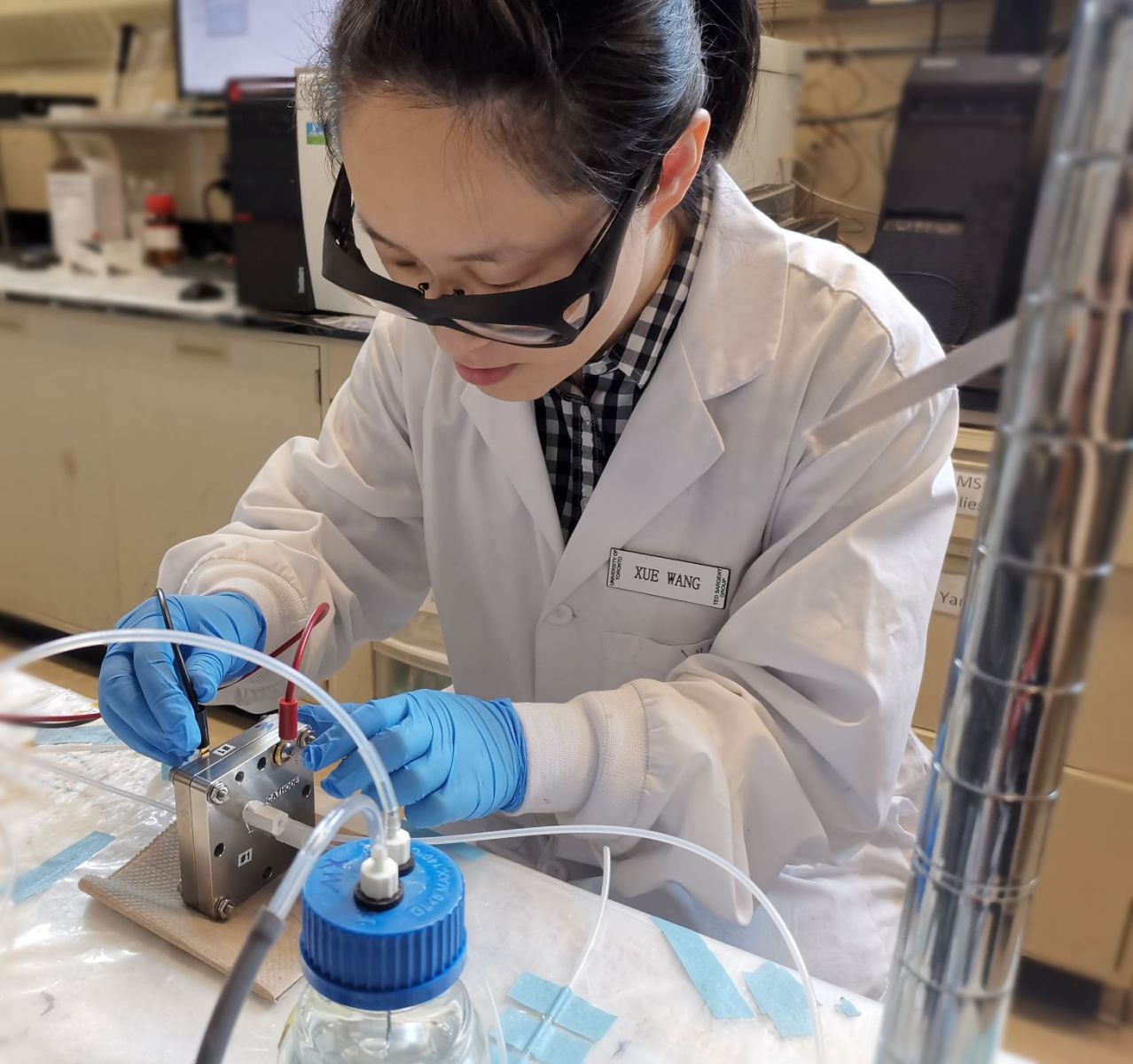
“We also found that the nitrogen-doped carbon layer is very important for producing ethanol because it can promote the reaction’s selectivity to ethanol,” said Xue Wang, a researcher in Prof. Ted Sargent’s group.
Wang explained that ethylene and ethanol, two valuable chemicals that can be created from CO2, are derived from a shared key intermediate (HOCCH*) in the conversion reaction, but the end result depends on whether C-O bond-breaks from HOCCH*, which results in ethylene, or stay stable, which results in the desired product ethanol.

“Our goal is to suppress the C-O bond breaking from HOCCH* so less ethylene will be produced. We found that this catalyst is very efficient for creating ethanol,” Wang said.
The researchers also discovered that the N-C/Cu catalyst is a strong and stable system, which is necessary for potential commercialization. In their experiments, they achieved 15 hours of stability at 52 percent Faradaic efficiency (FE) with a full cell energy efficiency (EE) of 16% in creating ethanol. The researchers aim to further improve the selectivity to ethanol, production rate, EE, concentration of ethanol and operational stability in order for the system to be used successfully in industrial applications.
Wang, Xue, Ziyun Wang, F. Pelayo García de Arquer, Cao-Thang Dinh, Adnan Ozden, Yuguang C. Li, Dae-Hyun Nam et al. "Efficient electrically powered CO 2-to-ethanol via suppression of deoxygenation." Nature Energy 5, no. 6 (2020): 478-486. DOI: 10.1038/s41560-020-0607-8.
For more information, contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
