Researchers make connection between the oceans' organic carbon and iron
Researchers from Concordia University now have a better understanding of the world’s largest carbon sink - the oceans.
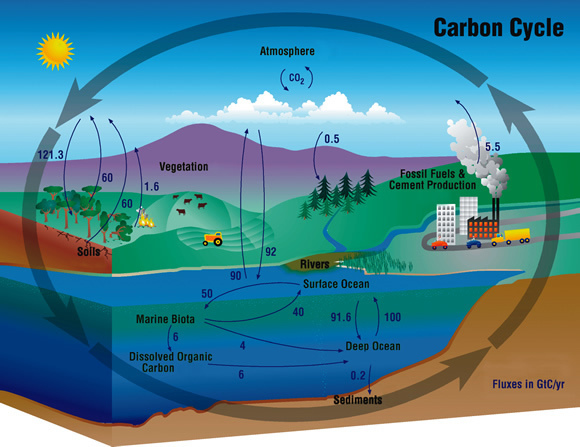
By Pippa WysongDiagram of the carbon cycle. Courtesy of NASA.
Concordia University researchers have a better understanding of why the world’s oceans are the largest carbon sink as a result of organic carbon remaining on the ocean floor rather than being released into the atmosphere as carbon dioxide.

“When carbon and iron react and combine in certain ways, they create stable compounds which can persist for thousands of years in ocean floor environments, keeping carbon stored in the sediment,” says Dr. Yves Gélinas, professor of chemistry at Concordia University in Montreal.
As the largest sink for organic carbon, oceans have the potential to be part of the solution to reducing the impact of global warming rather than adding to the problem.
Organic carbon comes from living matter. In the ocean, this is mostly through plankton that processes small inorganic carbon compounds. When plankton cells die, they sink and some of their remains, which are now rich with organic carbon end up on the ocean floor.
Why these carbon reservoirs don’t easily break down and cycle back into the atmosphere as carbon dioxide isn’t fully understood, but the Concordia University researchers have more answers after studying ocean sediment samples, using the intensely bright light of the Canadian Light Source.
Dr. Gélinas and Concordia PhD student Andrew Barber analyzed the chemical interactions between the iron and the organic carbon. Iron is responsible for keeping about 30 per cent of carbon that’s buried in ocean sediment in place. They wanted to determine how organic carbon reacts with iron in ocean sediment as this is the key that tells the story of how carbon stays in certain locations. Unlike the strong bonds created between iron and organic carbon in the world’s oceans, other types of reactions create bonds that are weaker and not as stable.
The researchers used the synchrotron to analyse a dozen samples of ocean floor sediment collected from the Antarctica, the equatorial Pacific, the St. Lawrence River estuary, and the Black Sea – environmentally diverse locations where the chemistry behaves differently.
They found that iron and organic carbon formed more stable compounds in sediment samples from coastal regions than those from deep sea areas. This has a lot to do with the greater amount of plankton and the chemistry related to its decomposition in coastal areas, says Dr. Gélinas.
From this information, the researchers created a model to explain the iron-organic carbon reaction mechanism, which may eventually be used as part of global carbon cycle models.
Barber, Andrew, Jay Brandes, Alessandra Leri, Karine Lalonde, Kathryn Balind, Sue Wirick, Jian Wang, and Yves Gélinas. "Preservation of organic matter in marine sediments by inner-sphere interactions with reactive iron." Scientific Reports 7 (2017). DOI: 10.1038/s41598-017-00494-0
To arrange an interview, contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
Suggested cutline for photo:
Andrew Barber and Dr. Yves Gélinas with lab members Kathryn Balind, Aleshia Kormendi, and Anic Imfeld (l to r) onboard the R/V Coriolis II operated by Reformar while on tour in the St. Lawrence Estuary and Gulf, as well as in the Saguenay Fjord. Photo: Dr. Yves Gélinas.
